Yang-Rae Kim, Stanley C. S. Lai, Kim McKelvey, Guohui Zhang, David Perry, Thomas S. Miller, Patrick R. Unwin*
Journal of Physical Chemistry C 2015, 119(30), 17389−17397
Publication online: June 30, 2015
Publication date: July 30, 2015
DOI: 10.1021/acs.jpcc.5b03513
ISSN: 1932-7447
Journal country: United States
Publisher: AMER CHEMICAL SOC
URL: http://pubs.acs.org/doi/abs/10.1021/acs.jpcc.5b03513
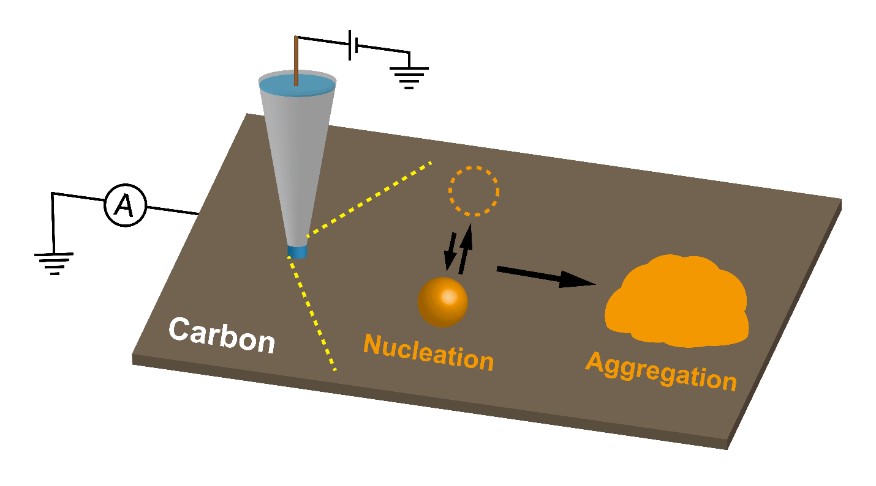
Abstract: The mechanism and kinetics of the electrochemical nucleation and growth of palladium (Pd) nanoparticles (NPs) on carbon electrodes have been investigated using a microscale meniscus cell on both highly oriented pyrolytic graphite (HOPG) and a carbon-coated transmission electron microscopy (TEM) grid. Using a microscale meniscus cell, it is possible to monitor the initial stage of electrodeposition electrochemically, while the ability to measure directly on a TEM grid allows subsequent high-resolution microscopy characterization which provides detailed nanoscopic and kinetic information. TEM analysis clearly shows that Pd is electrodeposited in the form of NPs (approximately 1–2 nm diameter) that aggregate into extensive nanocrystal-type structures. This gives rise to a high NP density. This mechanism is shown to be consistent with double potential step chronoamperometry measurements on HOPG, where a forward step generates electrodeposited Pd and the reverse step oxidizes the surface of the electrodeposited Pd to Pd oxide. The charge passed in these transients can be used to estimate the amounts of NPs electrodeposited and their size. Good agreement is found between the electrochemically determined parameters and the microscopy measurements. A model for electrodeposition based on the nucleation of NPs that aggregate to form stable structures is proposed that is used to analyze data and extract kinetics. This simple model reveals considerable information on the NP nucleation rate, the importance of aggregation in the deposition process, and quantitative values for the aggregation rate.
Download: 25_Journal of Physcial Chemistry C.pdf

 26. Time-resolved detection and analysis of single nanopartic...
26. Time-resolved detection and analysis of single nanopartic...
 24. Redox-dependent spatially resolved electrochemistry at gr...
24. Redox-dependent spatially resolved electrochemistry at gr...



















