Yang-Rae Kim†, R. Soyoung Kim†, Sun Kil Kang, Myung Gil Choi, Hong Yeong Kim, Daeheum Cho, Jin Yong Lee*, Suk-Kyu Chang*, Taek Dong Chung*
[† contributed equally]
Journal of the American Chemical Society 2013, 135(50), 18957−18967
Publication online: November 25, 2013
Publication date: December 18, 2013
DOI: 10.1021/ja410406e
ISSN: 0002-7863
Journal country: United States
Publisher: AMER CHEMICAL SOC
URL: http://pubs.acs.org/doi/abs/10.1021/ja410406e
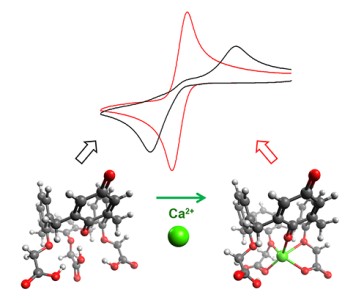
Abstract: Calix[4]arene-triacid-monoquinone (CTAQ), a quinone-containing water-soluble ionophore, was utilized to investigate how proton-coupled electron transfer (PCET) reactions of quinones were influenced by redox-inactive metal ions in aqueous environment. This ionophoric quinone derivative captured a Ca2+ ion that drastically altered the voltammetric behavior of quinone, showing a characteristic response to pH and unique redox wave separation. Spectroelectrochemistry verified significant stabilization of the semiquinone, and electrocatalytic currents were observed in the presence of Ca2+-free CTAQ. Using digital simulation of cyclic voltammograms to clarify how the thermodynamic properties of quinones were altered, a simple scheme was proposed that successfully accounted for all the observations. The change induced by Ca2+ complexation was explained on the basis of the combined effects of the electrostatic influence of the captured metal ion and hydrogen bonding of water molecules with the support of DFT calculation.
 21. Tunable decoration of reduced graphene oxide with Au nano...
21. Tunable decoration of reduced graphene oxide with Au nano...
 19. Electrokinetic concentration on a microfluidic chip using...
19. Electrokinetic concentration on a microfluidic chip using...
















