Sharel P. E, Yang-Rae Kim, David Perry, Cameron L. Bentley, and Patrick R. Unwin*
ChemElectroChem 2016, 3(12), 2189−2195
Publication online: October 14, 2016
Publication date: December 1, 2016
DOI: 10.1002/celc.201600404
ISSN: 2196-0216
Journal country: Germany
Publisher: WILEY-V C H VERLAG GMBH
URL: https://pubs.acs.org/doi/abs/10.1021/acsami.6b10940
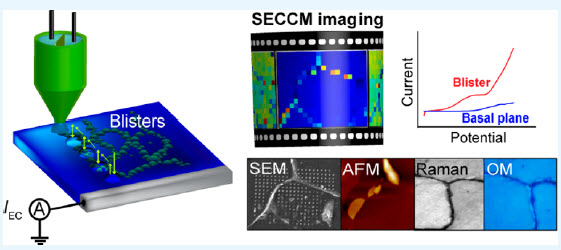
Abstract: There is great interest in finding and developing new, efficient, and more active electrocatalytic materials. Surface modification of highly oriented pyrolytic graphite, through the introduction of surface “blisters”, is demonstrated to result in an electrode material with greatly enhanced electrochemical activity. The increased electrochemical activity of these blisters, which are produced by electro-oxidation in HClO4, is revealed through the use of scanning electrochemical cell microscopy (SECCM), coupled with complementary techniques (optical microscopy, field emission-scanning electron microscopy, Raman spectroscopy, and atomic force microscopy). The use of a linear sweep voltammetry (LSV)-SECCM scan regime allows for dynamic electrochemical mapping, where a voltammogram is produced at each pixel, from which movies consisting of spatial electrochemical currents, at a series of applied potentials, are produced. The measurements reveal significantly enhanced electrocatalytic activity at blisters when compared to the basal planes, with a significant cathodic shift in the onset potential of the hydrazine electro-oxidation reaction. The improved electrochemical activity of the hollow structure of blistered graphite could be explained by the increased adsorption of protonated hydrazine at oxygenated defect sites, the ease of ion–solvent intercalation/deintercalation, and the reduced susceptibility to N2 nanobubble attachment (as a product of the reaction). This study highlights the capability of electrochemistry to tailor the surface structure of graphite and presents a new electrocatalyst for hydrazine electro-oxidation.